Contents
The States of Matter
A simple definition of matter is the “stuff” that takes up space and has mass.
Matter is made up of atoms. Atoms are made up of protons, neutrons and electrons. Atoms come together to form molecules which are the building blocks of every thing in the universe.


Most thing around you are made of atoms, millions and millions of atoms.

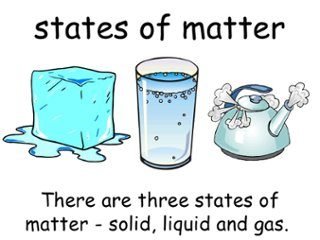
There are three basic states of matter matter: solids, liquids, and gases.
Solids
Solids refer to “stuff” that have a definite shape that does not conform to the container it is placed. The particles in a solid are tightly packed together and don’t move much.

Liquids
Liquids can flow. Its particles are loosely packed together and can move about. Liquid shape can change because it does not conform to the container it is placed.

Gas
Gas has no fixed shape or volume. There is much space between the particles in a gas. The particles also have a lot of kinetic energy (energy in motion). This means the particles move around and may collide with each other. The particles then spread around and can fill a container. Within the container, the particles exert a force on the interior of the container. This force is called pressure.


Solid to Liquid and Back to Solid
Energy in the form of heat is the easiest way to help a solid change to a liquid.
Melting and Freezing
By now you should notice that matter can change from one state to another. The physical state of matter changes when energy is removed or added to the matter.
When heat is applied to a solid its particles vibrate faster and mover farther apart. When a certain combination of temperature and pressure is applied, the solid will melt and turn into a liquid. Thus for water, the temperature needs to be a little over zero degrees Celsius to melt.

However, if you put ice in a glass of water and leave it out at room temperature, the ice will eventually come to the same temperature. The ice will melt (fusion) from the heat coming from the water.

As heat is removed from a liquid, the particles slow down and settle into one location. The liquid will become a solid.
It is important to note that the freezing point of liquid to solid is zero degrees Celsius or 32 degrees Fahrenheit. The melting point of sugar, salt, and rock is higher than that of water.


Sublimation and Vaporization
Sublimation occurs when a solid becomes a gas without gradually going through the process solid to liquid to gas.
Dry ice is a solid carbon dioxide. It will turn into a gas when left outside within 5 hours.
Vaporization occurs when a liquid becomes a gas through evaporation or boiling.
Condensation and Deposition
Condensation occurs when a gas loses energy and becomes a liquid.


Deposition occurs when a gas transforms directly into a solid.

Dry ice is a solid form of carbon dioxide. The process when dry ice changes from a solid to a gas, without turning into a liquid, is called sublimation. The opposite process is called deposition, when carbon dioxide changes from a gas to solid (dry ice).
Video: Change of State
Video: Effects of Temperature and Pressure on Matter
Review Chemistry Terms and Phase Change
- Fusion/Melting – solid to liquid
- Freezing – liquid to solid
- Vaporization/Boiling – liquid to gas
- Condensation – gas to liquid
- Sublimation – solid to gas
- Deposition – gas to solid
Worksheets
Click on the links below to download the PDF worksheets.


